Determine the Value and Units of the Rate Constant
We can use any two of the three. Question 12 Use the following information for questions 12-14 The following data were collected for the reaction.
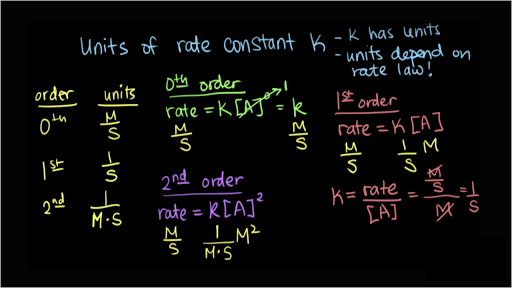
Units Of The Rate Constant Video Khan Academy
By seeing how the initial rate changes when we change the concentration of ceNOBr we can determine the value of x.

. A and b are the order of the reaction. For a zero order reaction the rate constant has units molar per second Ms or mole per liter per second molL 1 s 1 For a first order reaction the rate constant has units of per second of s -1. The rate equation relates mathematically the rate of reaction to the concentration of the reactants.
Rate Constant k has UNITS. The unit for the rate constant is calculated from the rate law. Now solve for the units of k.
Shields demonstrates how to calculate the value for the rate constant with units after determining the orders of each reactant using initial rates expe. Determine the value and units of the rate constant. First Order Reactions rate kA Mt k M k units.
First week only 499. Second Order Reactions rate kA 2 rate kAB Mt k M 2 k units. For the given rate law determine the units of the rate constant for rate k A 2 B.
M-1 s-1 M-1 min-1 M-1 hr-1 etc. 𝑘 667 10 30 units. Hence it is expressed in many units.
Solution for b Calculate the value of the rate constant k for the reaction. The rate law is textrate kceNOBrx where k is some constant and x is the order of the reaction in respect to ceNOBr. Thus gas constant R value can be given as Gas constant R 8314459848 Jmol 1 K 1.
The digits inside the parentheses are the uncertainty in the measurement of gas constant value. Third Order Reactions rate kA 3 rate kA 2 B rate kABC. Ea is the activation energy usually in kJmol.
A is the frequency factor which has the same units as the rate constant. Im going to use the first and third trials. In general for a reaction with order a b the units of the rate constant are mol 1 mn L mn1 s 1.
The relation between the rate constant and temperature is something you should be getting to know really well at this point. The reaction rate can depend on how concentrated our reactants are. At constant temperature the value of the equilibrium constant of a reversible reaction is constant and fixed.
Write the rate law for this reaction. Where k is the rate constant. R a t e A a B b.
Ms Mmin Mhr etc. A and B are the molar concentration of reactants A and B. A is the molar concentrations of substances A in moles per unit volume of solution.
R kAmBn r is used as symbol for rate The unit of r is usually mol dm-3 s-1 The square brackets A means the concentration of A unit mol dm-3 k is called the rate constant. In this video well use initial rates data to determine the rate law overall order and rate constant for the reaction between nitrogen dioxide and hydrogen gas. 1883 rate Δ A Δ t or rate k A The proportionality between the rate and A becomes an equal sign by the insertion of a constant k.
A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. MolL -1s -1 k molL -1 2 therefore k units are Lmol -1s -1 Top. This is the value in the rule-of-thumb often used.
Rate k A 2 the units of rate are molL -1s -1 and the units of A concentration are always molL -1 units have to be the same on each side. Following are the ways to express rate constant. The value of the gas constant R is 831 J K-1 mol-1.
Rate constant from the Arrhenius equation. K AeEaRT what is the equation called k is the rate constant. Select one answer o points 2 CH gCH1209 Time s ICHgICM 120 240 360 480 0530 0475 0431 0394 0363 Determine the rate equation question 12 the rate constant question 13 and the unit for the.
By raising the temperature just a little bit to 303 K this increases. A rate study of this reaction was conducted at 298 K. Like the rate constant the value of equilibrium constant also does not depend on the concentrations or pressures of the reactants and products.
At 20C 293 K the value of the fraction is. Rate kH 2 c. Start your trial now.
Determine the value and units of the rate constant k. Gas Constant In Different Units. SO 2 O 3 SO 3 O 2.
The gas constant is inversely used in diverse disciplines. Plug and chug using the rate law data from expt 1 and solving for k we get k 00427 s-1 7. The expression for the rate of the reaction can be shown as follows.
Include the correct units. Zero Order Reactions rate kA 0 Mt k k units. The presence of a catalyst changes the value of the rate constant.
B is the molar concentrations of substances B in moles per unit volume of solution. R is the reaction rate. The rate law for a chemical reaction can be determined using the method of initial rates which involves measuring the initial reaction rate at several different initial reactant concentrations.
Formula to calculate rate constant. The minute unit of all existing elements solid liquid as well as gas. That causes the rate of reaction to almost double.
You can see that the fraction of the molecules able to react has almost doubled by increasing the temperature by 10C. S-1 min-1 hr-1 etc. For the following reaction aA bB products the generalised rate equation is.
Mn are the partial orders of reaction. A chemical reactions rate law is an equation that describes the relationship between the concentrations of reactants in the reaction and the reaction rate. K A e E a R T.
K T is the reaction rate constant that depends on temperature. In the standard form the rate law equation is written as.

Watts W To Kilovolt Ampere Kva Kilowatts Kw Conversion Calculator Generators Zone Conversion Calculator Ampere Energy Symbols

Constant Proportionality Missing Values Tables Relationship Worksheets Math Practice Worksheets Proportional Relationships

6th Grade Math 6 G 4 Two Lesson Plans Middle School Math Resources Sixth Grade Math Math

How To Determine The Units Of The Rate Constant K Chemical Kinetics Youtube

How To Find The Rate Law And Rate Constant K Youtube

16 Pages This Mini Unit 3 Days Introduces The Y Mx B Form As A General Formula For Linear Fun Writing Linear Equations Introductory Algebra Teaching Algebra

Determining Rate Laws And The Order Of Reaction General Chemistry Jove

How To Use Hlookup Formula In Excel Excel Excel Formula Excel Hacks

Determining A Rate Law Using Initial Rates Data Worked Example Video Khan Academy

Middle School Math Foldable Notes Rates Proportionality Grade 7 Set 1 Middle School Math Math Foldables Math Foldables Middle School

How To Calculate A Rate Constant Recruitment Agencies Life Science Recruitment

Units Of Pressure Chemistry Notes Chemistry Worksheets Chemistry Notes Teaching Chemistry

Rate Of Change Activity Task Cards Relationship Worksheets 8th Grade Math Task Cards

Revenue Streams Powerpoint Presentation Powerpoint Presentation Powerpoint Revenue Streams

Units For K In Rate Law What Are The Units Of K In The Following Rate Law Rate K X Lisbdnet Com
Units Of The Rate Constant Video Khan Academy

Impairment Cost Meaning Benefits Indicators And More Money Management Advice Accounting And Finance Bookkeeping Business

Rational Functions And Equations Algebra 2 Unit Rational Function Algebra Lesson Plans Algebra Lessons
Comments
Post a Comment